The site is intended for US Healthcare Professionals only.
The site is intended for US Healthcare Professionals only.
SYNAGIS is indicated for the prevention of serious lower respiratory tract disease caused by respiratory syncytial virus (RSV) in pediatric patients:
SYNAGIS is not a vaccine. SYNAGIS is a monoclonal antibody that provides seasonal immunoprophylaxis.1
In preterm infants ≤35 wGA and children with and without BPD, SYNAGIS significantly reduced hospitalizations associated with severe RSV disease by more than half.2
57% fewer RSV-related ICU admissions
(3.0% vs 1.3%; P=0.026)
42% fewer total days of RSV-related hospitalizations
(per 100 children) (62.6 days vs 36.4 days; P<0.001)
40% fewer total days with increased supplemental oxygen
(per 100 children) (50.6 days vs 30.3 days; P<0.001)
There was no statistically significant difference in incidence (0.2% vs 0.7%) or total number of days per 100 children (1.7 days vs 8.4 days) of mechanical ventilation, incidence (14% vs 13%) or total days per 100 children (118 days vs 88 days) of hospitalization for non-RSV respiratory illness, or incidence of otitis media (40% vs 42%)2


*Reported events in at least 1% of children in the palivizumab group are provided along with the corresponding incidence in the placebo group. These represent adverse events reported by the investigator and include those identified by protocol-mandated testing and other clinically indicated evaluations.
There was no statistical significance in adverse events between children who received SYNAGIS and those who received placebo2
In postmarketing experience, the adverse reactions occurring ≥10% and at least 1% more frequently than placebo are fever and rash1
Inform parents and guardians of the possible side effects of SYNAGIS and of the signs and symptoms of potential allergic reactions, and advise of appropriate actions.
In children ≤24 months with CHD, SYNAGIS showed significant reductions in RSV-related hospitalizations3
 Study Design
Study Design
56% fewer total days of RSV-related hospitalizations
(per 100 children) (129 days vs 57.4 days; P=0.003)
73% fewer total days with increased supplemental oxygen
(per 100 children) (101.5 days vs 27.9 days; P=0.014)
There was no statistically significant difference in incidence of RSV-related ICU admission (3.7% for placebo vs 2.0% for SYNAGIS), total days of RSV-related ICU stay per 100 children (71.2 days for placebo vs 15.9 days for SYNAGIS), incidence of RSV-related mechanical ventilation (2.2% for placebo vs 1.3% for SYNAGIS), or total days of RSV-related mechanical ventilation per 100 children (54.7 days for placebo vs 6.5 days for SYNAGIS)
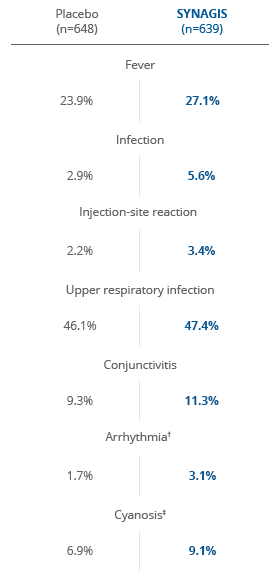
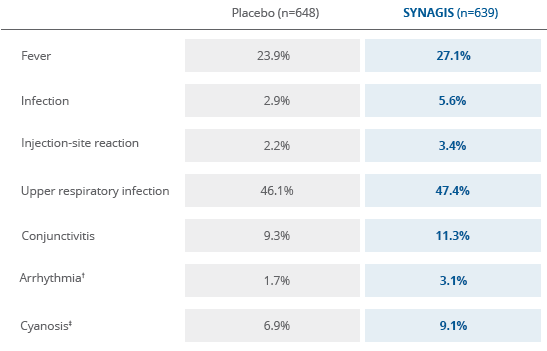
†Few adverse events were reported at an absolute incidence >1% higher in the SYNAGIS group compared with the placebo group.
‡None of these events reported as arrhythmia and one reported as cyanosis (placebo recipient) were judged related to the study drug.
There was no statistically significant difference in adverse events between children with CHD who received SYNAGIS and those who received placebo3
In postmarketing experience, the adverse reactions occurring ≥10% and 1% more frequently than placebo are fever and rash1
A 2013 clinical study published in the New England Journal of Medicine also showed that SYNAGIS significantly reduced RSV-related hospitalizations in late-preterm infants 33-35 wGA6
Serious adverse events reported as reason for hospitalization regardless of causation6


RSV = respiratory syncytial virus

SYNAGIS, 50 mg and 100 mg for injection, is indicated for the prevention of serious lower respiratory tract disease caused by respiratory syncytial virus (RSV) in pediatric patients:
SYNAGIS, 50 mg and 100 mg for injection, is indicated for the prevention of serious lower respiratory tract disease caused by respiratory syncytial virus (RSV) in pediatric patients:
The safety and efficacy of SYNAGIS have not been established for treatment of RSV disease.
Previous significant hypersensitivity reaction to SYNAGIS.
Hypersensitivity Reactions: Anaphylaxis and anaphylactic shock (including fatal cases) and other severe acute hypersensitivity reactions have been reported. Permanently discontinue SYNAGIS and administer appropriate medication if such reactions occur.
Coagulation Disorders: SYNAGIS should be given with caution to children with thrombocytopenia or any coagulation disorder.
RSV Diagnostic Test Interference: Palivizumab may interfere with immunological-based RSV diagnostic tests, such as some antigen detection-based assays.
Serious Adverse Reactions: The most common serious adverse reactions occurring with SYNAGIS are anaphylaxis and other acute hypersensitivity reactions.
Most Common Adverse Reactions: The most common adverse reactions are fever and rash.
Postmarketing Experience: Severe thrombocytopenia and injection site reactions have been identified during post approval use of SYNAGIS.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
These are not all the possible risks associated with SYNAGIS.
REFERENCE: 1.REFERENCES: 1. SYNAGIS [package insert]. Waltham, MA: Sobi, Inc.
2. The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102(3):531–537.
3. Data on file, 3110106, AstraZeneca Pharmaceuticals LP.
4. Sánchez PJ. Immunoprophylaxis of respiratory syncytial virus disease. Pediatr Infect Dis J. 2000;19(8):791–801.
5. Feltes TF, Cabalka AK, Meissner HC, et al; for Cardiac SYNAGIS Study Group. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143(4):532–540.
6. Blanken MO, Rovers MM, Molenaar JM, et al. N Engl J Med. 2013;368(19):1791–1799.
REFERENCES: 1. Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143(suppl 5):S127-S132.
2. Zhou H, Thompson WW, Viboud CG, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993-2008. Clin Infect Dis. 2012;54(10):1427-1436.
3. Centers for Disease Control and Prevention. Respiratory syncytial virus activity—United States, July 2011–January 2013. MMWR. 2013;62(8):141–144.
4. Panozzo CA, Fowlkes AL, Anderson LJ. Variation in timing of respiratory syncytial virus outbreaks: lessons from national surveillance. Pediatr Infect Dis J. 2007;26(suppl 11):S41–S45.
5. Centers for Disease Control and Prevention. Brief report: respiratory syncytial virus activity—United States, 2005–2006. MMWR. 2006;55(47):1277–1279.
6. Centers for Disease Control and Prevention. Brief report: respiratory syncytial virus activity—United States, July 2008–December 2009. MMWR. 2010;59(8):230–233.
7. Molinari-Such M, García I, García L, et al. Respiratory syncytial virus-related bronchiolitis in Puerto Rico. P R Health Sci J. 2005;24(2):137–140.
8. Panozzo CA, Stockman LJ, Curns AT, Anderson LJ. Use of respiratory syncytial virus surveillance data to optimize the timing of immunoprophylaxis. Pediatrics. 2010;126(1):116–123.
9. Hall CB. Respiratory syncytial virus: its transmission in the hospital environment. Yale J Bio Med. 1982;55(3–4):219–223.
REFERENCES: 1. Langston C, Kida K, Reed M, Thurlbeck WM. Human lung growth in late gestation and in the neonate. Am Rev Respir Dis. 1984; 129(4):607-613.
2. Yeung CY, Hobbs JR. Serum-gamma-G-globulin level in normal premature, post mature, and “small-for-dates” newborn babies. Lancet. 1968;1(7553):1167-1170.
3. Boyce TG, Mellen BG, Mitchel EF Jr, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr. 2000;137(6):865-870.
4. Ambrose CS, Anderson EJ, Simoes EAF, et al. Respiratory syncytial virus disease in preterm infants in the United States born at 32-35 weeks gestation not receiving immunoprophylaxis. Pediatr Infect Dis J. 2014;33(6):576-582.
REFERENCES: 1. Allen J, Zwerdling R, Ehrenkranz R, et al; American Thoracic Society. Statement on the care of the child with chronic lung disease of infancy and childhood. Am J Respir Crit Care Med. 2003;168(3):356–396.
2. Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357(19):1946-1955.
3. Boyce TG, Mellen BG, Mitchel EF Jr, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr. 2000;137(6):865-870.
4. Navas L, Wang E, de Carvalho V, Robinson J; and Pediatric Investigators Collaborative Network on Infection in Canada. Improved outcome of respiratory syncytial virus infection in a high-risk hospitalized population of Canadian children. J Pediatr. 1992;121(3):348-354.
REFERENCES: 1. Cabalka AK. Physiologic risk factors for respiratory viral infections and immunoprophylaxis for respiratory syncytial virus in young children with congenital heart disease. Pediatr Infect Dis J. 2004;23(suppl 1):S41-S45.
2. White MC. Anaesthetic implications of congenital heart disease for children undergoing non-cardiac surgery. Anaesth Intensive Care Med. 2009;10(10):504-509.
3. Boyce TG, Mellen BG, Mitchel EF Jr, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr. 2000;137(6):865-870.
4. Altman CA, Englund JA, Demmler G, et al. Respiratory syncytial virus in patients with congenital heart disease: a contemporary look at epidemiology and success of preoperative screening. Pediatr Cardiol. 2000;21(5):433-438.
STUDY DESIGN

A multicenter, randomized, placebo-controlled trial in infants born at ≤35 wGA or children with BPD (≤24 months of age) randomized (N=1502) to receive 1 injection of SYNAGIS (15 mg/kg) (n=1002) or an equal volume of placebo (n=500) every 30 days for a total of 5 doses.
A randomized, double-blind, placebo-controlled trial of 1287 children with hemodynamically significant CHD randomly assigned 1:1 to receive 5 monthly intramuscular injections of SYNAGIS 15 mg/kg or placebo.*
*Results may not be generalizable to a US population.
A multicenter, randomized, double-blind, placebo-controlled trial of SYNAGIS 15 mg/kg in healthy preterm infants 33 to 35 weeks GA ≤6 months of age (N=429) conducted in the Netherlands. The number of RSV-related hospitalizations was a secondary endpoint of the trial.*
*Results may not be generalizable to a US population.
A retrospective chart review designed to assess patterns of RSV prophylaxis in high-risk infants who were discharged home before the end of the 2012-2013 RSV season. A total of 1005 charts were reviewed, including those of 71 infants born at <32 weeks GA and 166 infants born at 32 to 34 weeks GA who were eligible to receive SYNAGIS and were discharged during the RSV season. The duration of RSV season was determined by the infant’s physician. Eligibility for RSV prophylaxis was based on the 2012 AAP guidelines.
A retrospective claims analysis of RSV hospitalization rates among commercially insured infants (N=5003) who received at least 1 dose of SYNAGIS between 2003 and 2009. Only infants discharged from birth hospitalization prior to RSV season and therefore eligible for treatment the whole RSV season were included. Compliance was defined as receiving ≥5 doses of SYNAGIS with no gaps between doses of >35 days and receiving the index doses of SYNAGIS by November 30.
Zhou et al: Weekly statewide hospital discharge data were obtained from the Healthcare Cost and Utilization Project inpatient databases from 13 states (Arizona, California, Colorado, Illinois, Iowa, Kansas, Maryland, Massachusetts, New Jersey, Oregon, South Carolina, Washington, and Wisconsin) that provided complete weekly records from 1993 through 2008. The number of influenza- and RSV-associated hospitalizations was calculated as the sum of hospitalizations with an ICD-9-CM influenza or RSV code listed, plus an estimate of the contribution of influenza or RSV to uncoded hospitalizations for respiratory and circulatory disease based on models that incorporated weekly influenza and RSV surveillance data as covariates.
Normal lungs were obtained from 42 infants (29 liveborn and 13 stillborn) younger than 1 month of age coming to autopsy at Winnipeg Children's Hospital, Winnipeg, Manitoba, over a period of 3 years. Infant age (expressed as gestational age plus postnatal age) ranged from 19 weeks gestation to 3 weeks postnatal age. The lung structures studied were air spaces (saccules or alveolar ducts and alveoli); air-space wall (alveolar and/or saccular); conducting air (lumen of bronchi and bronchioles); and nonparenchyma (all other structures).1
Random serum samples of 182 babies of normal weight for gestational age ranging from 24 to 40 weeks were studied. Blood samples were obtained within 24 hours of birth from heel pricks or indwelling umbilical catheters inserted for other purposes. Gestational age was estimated in the first place from the date of last menstrual period and later confirmed by neurological and electroencephalographic examination whenever possible.1
A retrospective study analyzed hospitalizations for RSV-related illness in children <3 years of age enrolled in the Tennessee Medicaid system from July 1989 through June 1993. Rates are based on expected hospitalizations per year per 1000 children
An observational prospective study of preterm infants 32 to 35 wGA not receiving prophylaxis who were <6 months of age as of November 1 (N=1642) conducted in 188 US outpatient clinics over 2 RSV seasons (2009-2010 and 2010-2011). Active testing for RSV was conducted using quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) testing.
A retrospective study analyzed hospitalizations for RSV-related illness in children <3 years of age enrolled in the Tennessee Medicaid system from July 1989 through June 1993. Rates are based on expected hospitalizations per year per 1000 children
A retrospective chart review of morbidity and mortality among patients identified with RSV by viral isolation or antigen detection at 12 tertiary care pediatric centers from 1988 to 1991. Children (N=1584) were included if they had lower respiratory tract infection and at least 1 of the following: congenital heart disease, chronic lung disease, immunosuppression, prematurity (<36 wGA), age <6 weeks, and oxygen saturation <90% or arterial oxygen pressure <60 mm Hg in room air.
A retrospective study analyzed hospitalizations for RSV-related illness in children <3 years of age enrolled in the Tennessee Medicaid system from July 1989 through June 1993. Rates are based on expected hospitalizations per year per 1000 children.
A retrospective review of the impact of RSV-related hospitalizations in 63 children with CHD over 2 consecutive RSV seasons (1994-1995 and 1995-1996) at a large, academic pediatric hospital. Patients with insignificant cardiac diagnoses (eg, innocent murmur, benign chest pain, and benign premature atrial contractions) were excluded.
For Colorado prescribers, visit synagishcp.com/wac-pricing.
The AAP recommends consideration of providing more than 5 consecutive doses of [SYNAGIS] depending on the duration of the current RSV surge in a given region of the country.
Looking for more information? We're happy to help you with concerns or questions you may have about SYNAGIS® (palivizumab) or severe RSV disease.
AstraZeneca Customer Support Network
Call toll-free:1-877-633-44111-877-633-4411
Hours of operation: Monday—Friday, 8:00 AM — 6:00 PM ET